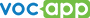First 20 Elements of the periodic table
0 20 schede annadube15
The periodic table of elements
The periodic table of elements, is a tabular arrangement of the chemical elements, ordered by their atomic number, electron configuration, and recurring chemical properties, whose structure shows periodic trends. Generally, within one row (period) the elements are metals to the left, and non-metals to the right, with the elements having similar chemical behaviours placed in the same column.Periods and groups
Table rows are commonly called periods and columns are called groups. Six groups have accepted names as well as assigned numbers: for example, group 17 elements are the halogens; and group 18 are the noble gases. Also, displayed are four simple rectangular areas or blocks associated with the filling of different atomic orbitals.Chemical elements numbers
Each chemical element of periodic table has a unique atomic number (Z) representing the number of protons in its nucleus. Most elements have differing numbers of neutrons among different atoms, with these variants being referred to as isotopes. The periodic table has 118 confirmed elements, from element 1 (hydrogen) to 118 (oganesson).First 20 elements of the periodic table
We have found an easy way to remember the names and symbols of the first 20 elements of the periodic table. You can learn the elements with the help of flashcards and make lessons in your convenient way. To make your pronunciation better we also have implemented audio clips in mp3 format.Here's a list of 20 elements ordered by increasing atomic number. Each element has one or two letter symbols, which is an abbreviated form of its present.
Although, 20 elements are not all of them, they are a great start if you think about chemistry seriously. Do you know the first 20 elements of the periodic table? Check yourself with our flashcard set!